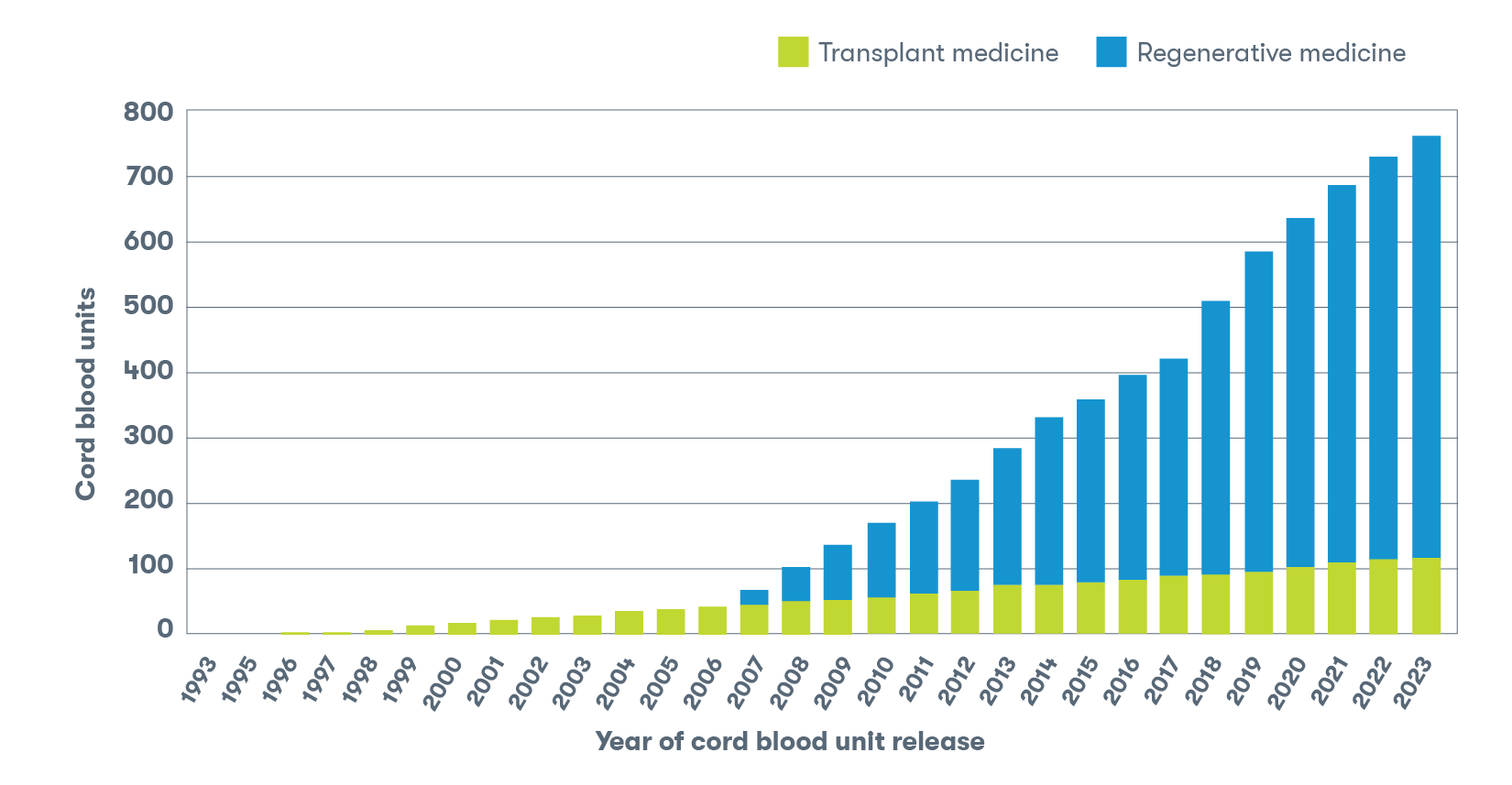
In honor of this milestone, we’re taking a comprehensive look at how hundreds of families have used their stored newborn stem cells, beginning with the very first cord blood sample released by the CooperSurgical laboratory in Tucson, in 1993.2
Cumulative cord blood units released
As part of the world’s largest and most experienced family newborn stem cell preservation organization, the team has released 760 cord blood samples intended for use in transplant and regenerative medicine, which is more than any other family bank, further solidifying our position as the world’s largest and most experienced family newborn stem cell preservation company.2
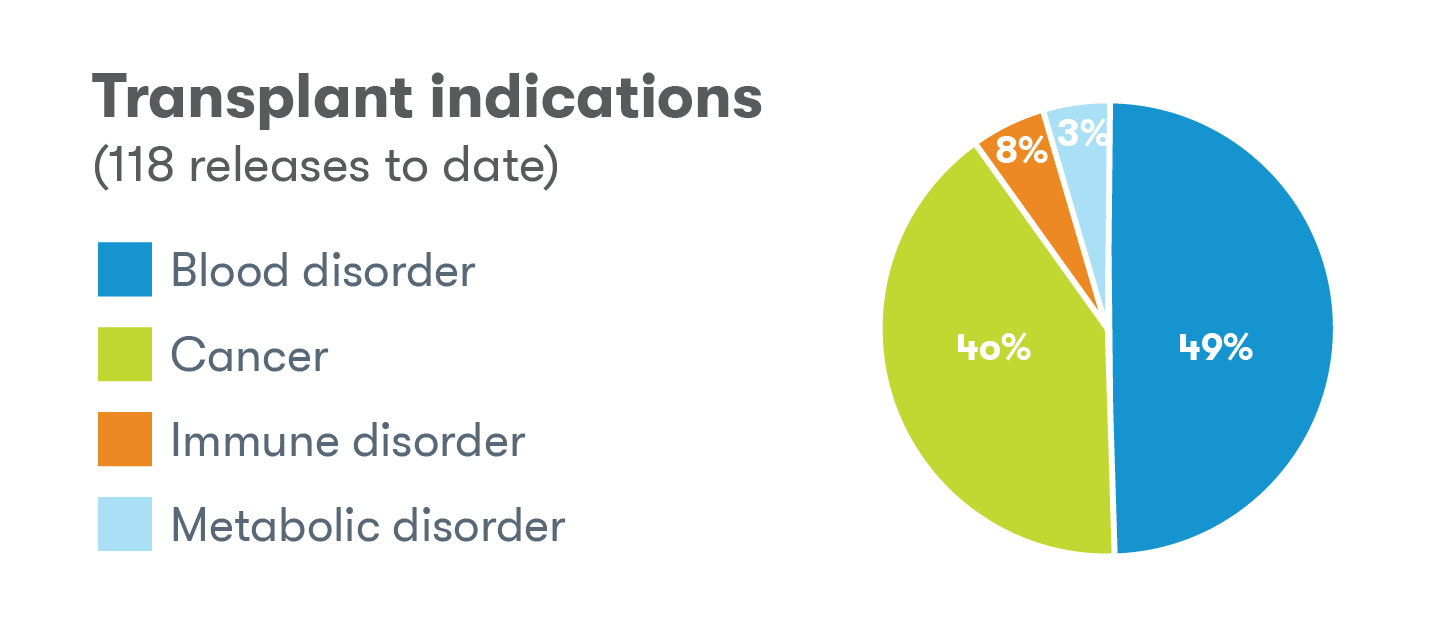
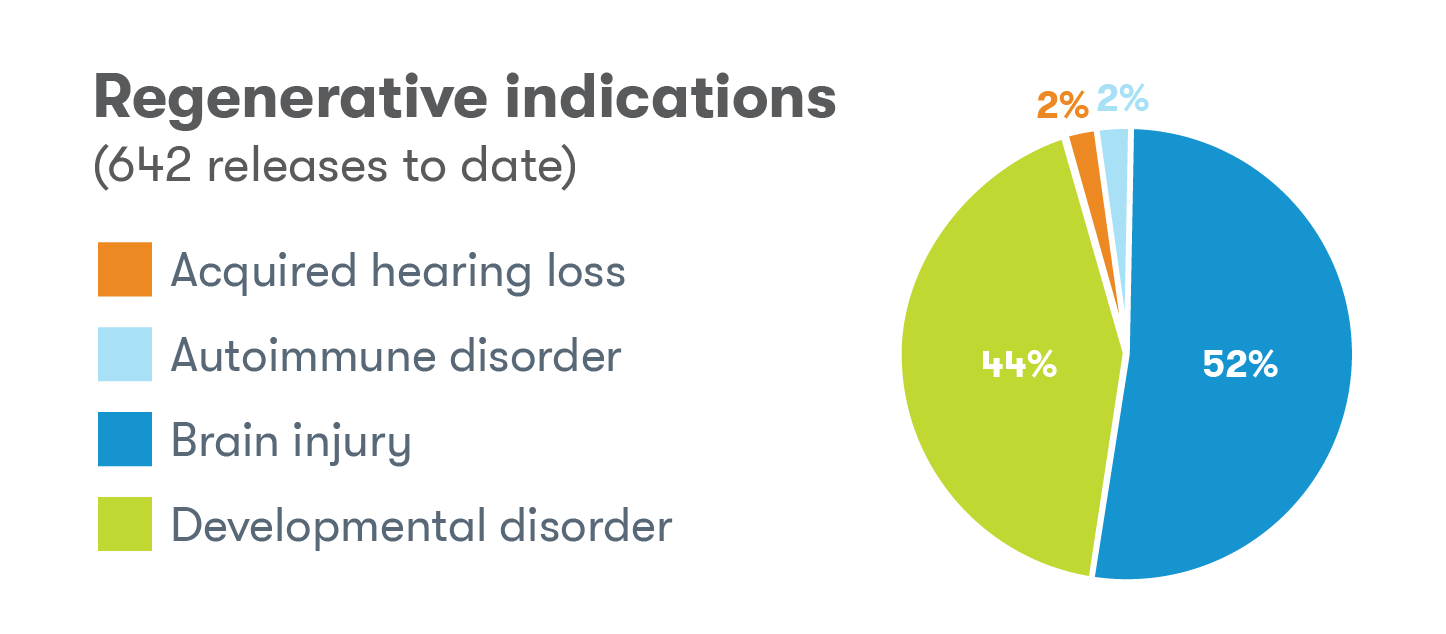
Indications for releases
The use of cord blood as a source of stem cells in transplant medicine was still in its relatively early stages back in 1993 when we released the first cord blood sample to a family for intended use in a stem cell transplant.2 Today, over 45,000 cord blood transplants have been performed worldwide3 and cord blood can be used to treat over 80 serious conditions.4 The Tucson laboratory has released for a number of these conditions for intended use, but some of the most common include sickle cell disease, acute lymphocytic/lymphoblastic leukemia (ALL), and beta thalassemia.2
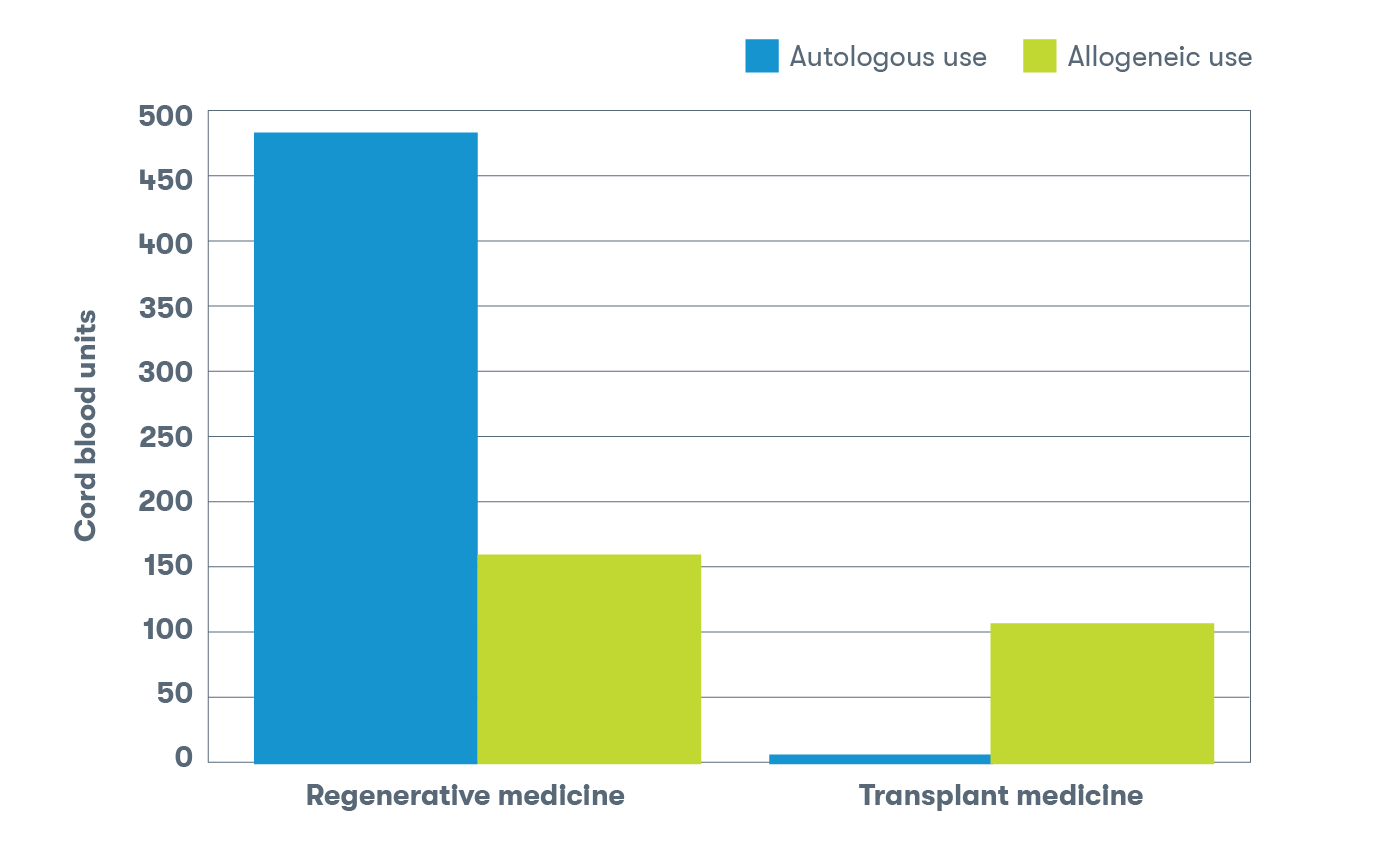
Autologous vs. allogeneic use
The decision regarding the use of autologous stem cells (patient’s own) or allogeneic stem cells (donor source) for a transplant procedure or regenerative medicine protocol depends on several factors. These factors include the specific condition being treated and the patient’s individual circumstances and medical history.5
Approximately 65% of releases from the Tucson laboratory were intended for procedures involving the patient’s own cord blood.2 Autologous use is more common for regenerative medicine indications, while allogeneic use is more common for stem cell transplants. The allogeneic uses include one syngeneic use, a rare occurrence in which the donor and recipient are identical twins.
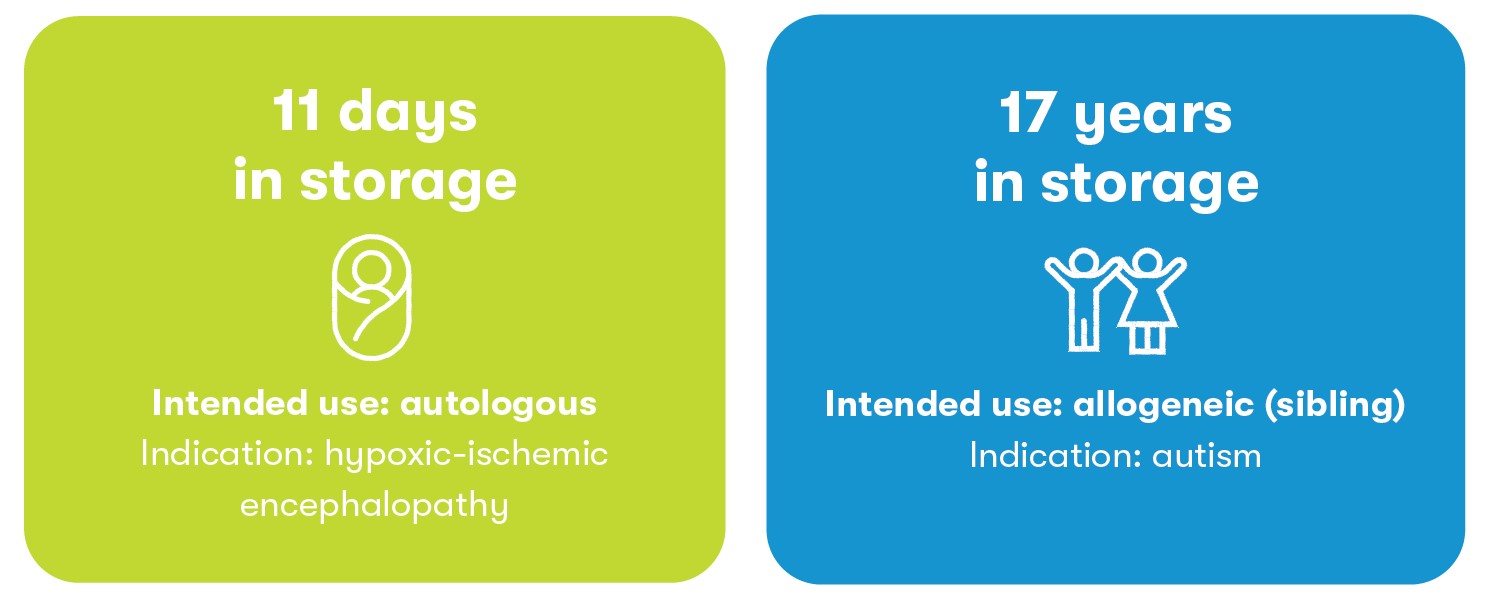
Age range of samples
Samples ranging in age from 11 days to 17 years old have been released by the Tucson laboratory.2 Proper cryostorage helps ensure that cord blood samples remain viable for many decades.6
A once-in-a-lifetime chance
If asked, we suspect the majority of families represented here would tell you that they never expected to need their child’s preserved newborn stem cells. However, when the need arose within their immediate family that called for a cord blood sample release, these parents were glad that this precious medical resource was available for their loved one.
Expecting a child, or have friends or family who are? How about a grandchild? Enroll with Cells for Life today or refer a friend. When someone you refer preserves with Cells for Life, you’ll receive a cheque or storage credit to fund your storage fees.*
1. Knudtzon S. Blood. 1974;43:357-361. 2. Internal data on file. 3. Wagner JE. Cord blood 2.0: state of the art and future directions in transplant medicine. Blood Res. 2019 Mar;54(1):7-9. doi: 10.5045/br.2019.54.1.7. Epub 2019 Mar 21. PMID: 30956957; PMCID: PMC6439299. 4. Mayani, H., Wagner, J.E. & Broxmeyer, H.E. Cord blood research, banking, and transplantation: achievements, challenges, and perspectives. Bone Marrow Transplant 55, 48–61(2020). https://doi.org/10.1038/s41409-019-0546-9. 5. Kindwall-Keller TL, Ballen KK. Umbilical cord blood: The promise and the uncertainty. Stem Cells Transl Med. 2020;9(10):1153-1162. 6. Broxmeyer HE, Lee MR, Hangoc G, et al. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood. 2011;117(18):4773-7.
*Refer-a-Friend Program: See website for referral terms and conditions.