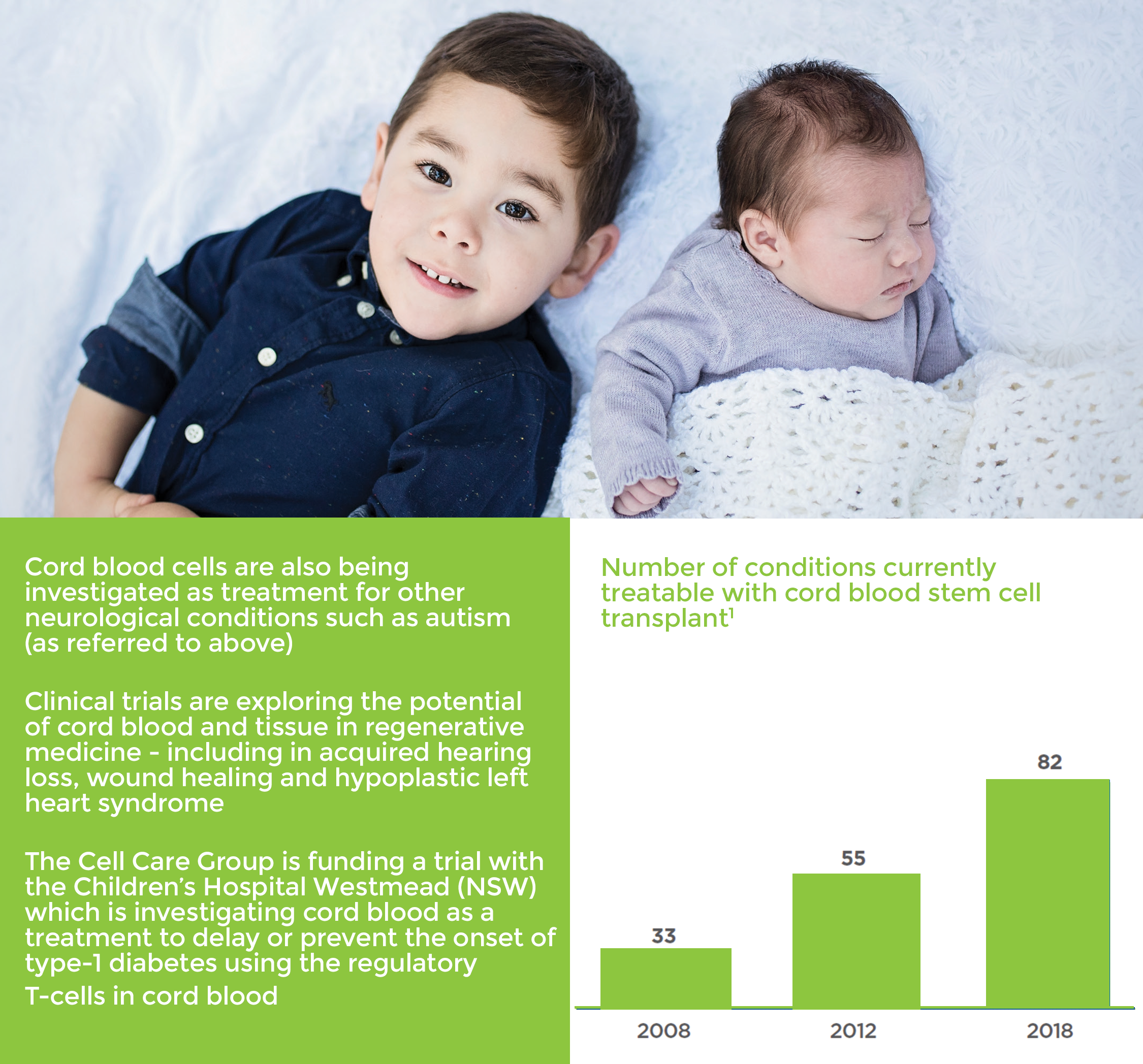
Umbilical cord blood is being investigated globally as a potential treatment for cerebral palsy.
The Cell Care Group, consisting of Cell Care in Australia and Cells for Life & Insception Lifebank in Canada, is collaborating with the Cerebral Palsy Alliance to fund a Phase 1 safety trial, led by Murdoch Children’s Research Institute (MCRI). It aims to determine whether sibling cord blood is safe, and as a secondary consideration, efficacious for children with cerebral palsy.
Brodie Munz was diagnosed with cerebral palsy at 18 months old. His symptoms were limited movement in his left side and development delays. Ben and Brenda, Brodie’s parents, chose to collect his newborn sister Zoey’s cord blood through a free CP sibling cord blood collection program. Brodie was one of the cord blood recipients in the MCRI Phase I trial. His treatment took place last December.
Since the cord blood infusion, Brenda and Ben have reported notable improvements in Brodie’s condition.
“Particularly with his left arm, since the cord blood treatment Brodie’s increased movement and strength in his left arm has had a significant impact on his quality of life. Prior to December, he would avoid playground apparatus requiring use of his left arm; it’s no longer the case now.”
Brenda says “I’ve even noticed a difference in his intellectual ability. He is definitely brighter and more engaging since the infusion.”
Professor Iona Novak, Head of Research at the Cerebral Palsy Alliance Research Institute said, “The CP sibling cord blood collection program that Brodie has benefited from is a significant contribution to cerebral palsy research in Australia. The clinical trial that Brodie has participated in is an important study that will add to the growing body of evidence assessing sibling cord blood infusion as a potential therapy for cerebral palsy.” Early-stage clinical studies have suggested that there may be improvements in motor function, such as that being described by Brenda and Ben, when a child’s own cord blood or unrelated cord blood has been infused in the setting of cerebral palsy.
Duke University is a world leader trialing cord blood therapy for children with CP and other neurological disorders. A recent publication investigated the impact of a child’s own cord blood (autologous) in CP. Professor Joanne Kurtzberg, chief investigator stated, “results of this trial suggest that when adequately dosed, an intravenous infusion of autologous umbilical cord blood improves whole brain connectivity and motor function in young children with cerebral palsy.”2
Based on safety and clinical benefits reported in cord blood trials, the FDA (in the US) has approved an expanded access protocol to enable access to sibling or a child’s own umbilical cord blood for treatment in children with various brain disorders, including cerebral palsy and autism. Families must have stored their child’s own cord blood, or that of a matched sibling, in a family bank. For more information on this program, click here.
Umbilical cord blood and tissue are rich in powerful stem cells that can only be collected at birth.
- https://nybloodcenter.org/products-and-services/blood-products/national-cord-blood-program/cord-blood-101/
- Effect of Autologous Cord Blood Infusion on Motor Function and Brain Connectivity in Young children with Cerebral Palsy: A Randomized, Placebo-Controlled Trial. Stem Cells Translational Medicine, 2017. Doi: 10.1002/sctm.17-0102